Mg s 2HCl aq MgCl 2 aq H 2 g. Also the equation is not balanced and the molecular formula of magnesium nitride is written wrong.

How To Balance Mg N2 Mg3n2 Youtube
Write the chemical equation for the.

. What is the balanced equation for the reaction of photosynthesis. The formula for magnesium nitride is Mg_3N_2. The answer will appear below.
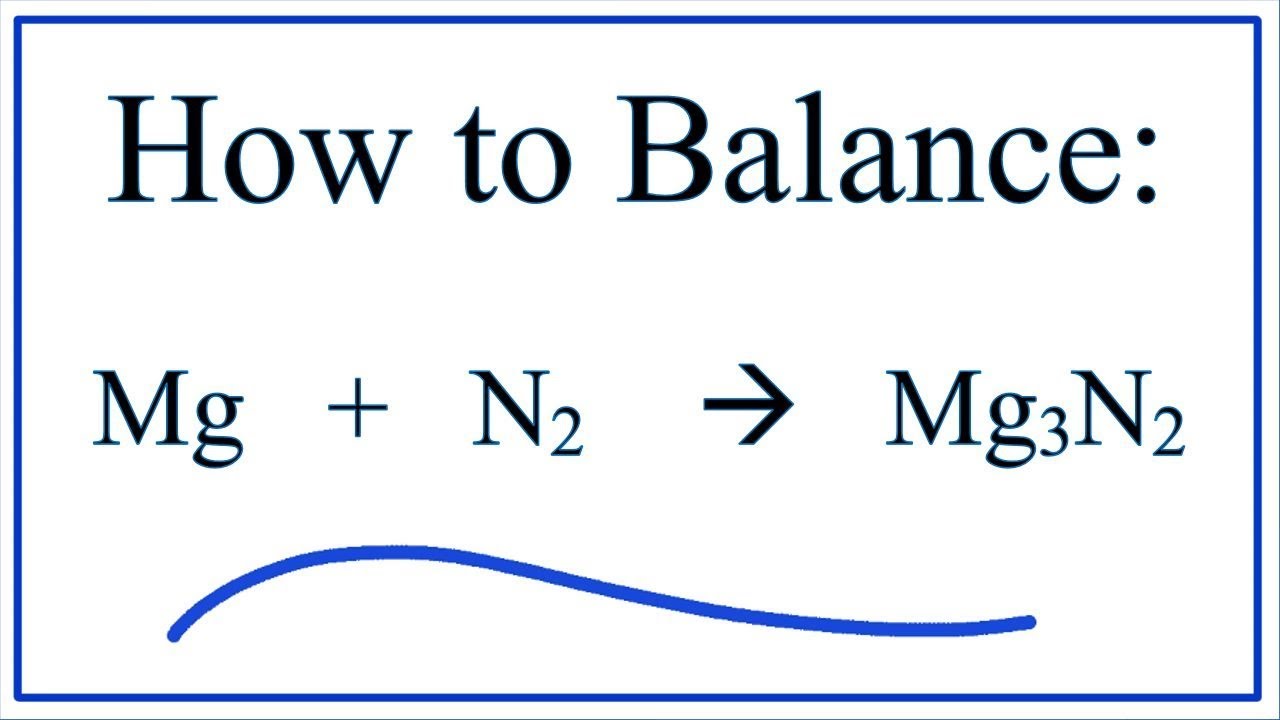
Mg 3 N 2 3Mg N 2. 1 2 2. Magnesium with molecular nitrogen c.
Write the chemical equation for the reaction of magnesium nitride with water to form magnesium oxide and ammonia. When magnesium is burned in air some of the magnesium also reacts with nitrogen in the air and some magnesium nitride is also formed. Fe Au Co Br C O N F.
3 M g N 2 Δ M g 3 N 2. What is the balanced equation for the chemical reaction Mg_3N_22H_2O - MgOH_2NH_3. 2 2 yes.
Mg_3N_2s 6H_2Ol rarr 3MgOH_2aq 2NH_3aq. Magnesium is classified as an alkaline earth metal and has 2 hydration shells. Empirical and Molecular Formulas 9.
Write the chemical equation for the reaction of magnesium with molecular nitrogen to form magnesium nitride. For this reaction we have a decomposition reaction. When magnesium react with nitrogen magnesium nitride is formed according to the following equation-3 Mg N2 ---- Mg3N2.
Calculate the empirical formula of a compound. Magnesium react with oxygen at room temperature forming a passivating layer of MgO on the surface. The above reaction can be a synthesis and a combination both as two reactants react to form a single product.
Co - cobalt and CO - carbon monoxide. Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. Reaction is given below.
To enter an electron into a chemical equation use - or e. The element can be found in abundance in the hydrosphere and in mineral salts such as dolomite and magnesium carbonateCommon dietary sources of magnesium include nuts cashews peanuts almonds beans bananas apples carrots broccoli and leafy greens. The reaction of nitric oxide and nitrogen dioxide at very low temperatures produces a product that is a deep blue solid.
Write correctly balanced chemical equations for the following reactions. Magnesium is an important. Write correctly balanced chemical equations for the following reactions.
Marine the chemical equation for the reaction of me 3. The chemical equation is3 Mg N2 Mg3N2. What is the balanced equation between magnesium nitrogen and magnesium nitride.
3Mgs N2g ----- Mg3N2s. A chemical equation is balanced using the law of conservation of mass which states that matter cannot be created nor destroyed Q8. What is the balanced equation between magnesium nitrogen and magnesium nitride.
The chemical formula for this reaction is Mg N2 MgN2. What will be the balanced equation for the reactionmagnesium burns in the presence of nitrogen to form magnesium nitride. Soobee72pl and 17 more users found this answer helpful.
N 2 O 2 N 2 O 5 unbalanced equation The balanced chemical equation is-2N 2 5O 2 2N 2 O 5. Youll need to be patient balancing this equation. Ammonium nitrate Nitrogen gas Oxygen gas Water.
The balanced chemical equation can be given as. Magnesium with molecular oxygen b. The chemical formula of.
Magnesium nitride with water d. The balanced chemical equation is. Always use the upper case for the first character in the element name and the lower case for the second character.
Enter an equation of a chemical reaction and click Balance. Magnesium reacts with nitrogen to form magnesium nitride. Chemistry questions and answers.
On what basis is a chemical equation balanced. Nitrogen exists as diatomic molecule and so the N is wrong it should be N2. When Nitric acid react with magnesium after reaction magnesium changed into magnesium nitrate and hydrogen is formed.
When ingited Mg reacts with both oxygen and nitrogen forming a mixture of magnesium oxide MgO and magnesium nitride Mg 3. This reaction takes place at a temperature of 700-1500C. Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide.
At room temperature and pressure Mg3N2 is a greenish yellow powder. Solution First write the unbalanced. 3Mg N_2 stackrelDeltararr Mg_3N_2 As a nitride it can by hydrolyzed by water to form magnesium hydroxide and ammonia gas.
NH 4 NO 3 N 2 O 2 H 2 O. When magnesium nitride is treated with warm water. Analysis of the product showed that it contains 368 nitrogen and 6325 oxygen.
As do many active metals magnesium nitride can be formed by heating the metal fiercely under dinitrogen gas. The chemical equation is3 Mg N2 Mg3N2. The correct balanced equation with the physical states will be.
These coefficients yield equal numbers of both H and O atoms on the reactant and product sides and the balanced equation is therefore. Mg HNO3 MgNO32 H2. The product of this reaction is Magnesium Nitride.
The thermal decomposition of magnesium nitride to produce magnesium and nitrogen. Mg3N2 6H2O----- 3Mg OH2 2NH3. In this decomposition reaction NH4NO3 is breaking apart decomposing into N2 O2 H2O.
The balanced equation for the reaction is 2Mg sO2 g 2MgO s When 102 g Mg is allowed to react with 106 g O2 118 g MgO is collected. Type of Chemical Reaction. Reaction of magnesium with air.
How To Balance Mg N2 Mg3n2 Youtube

Mg N2 Mg3n2 Balanced Chemical Equation
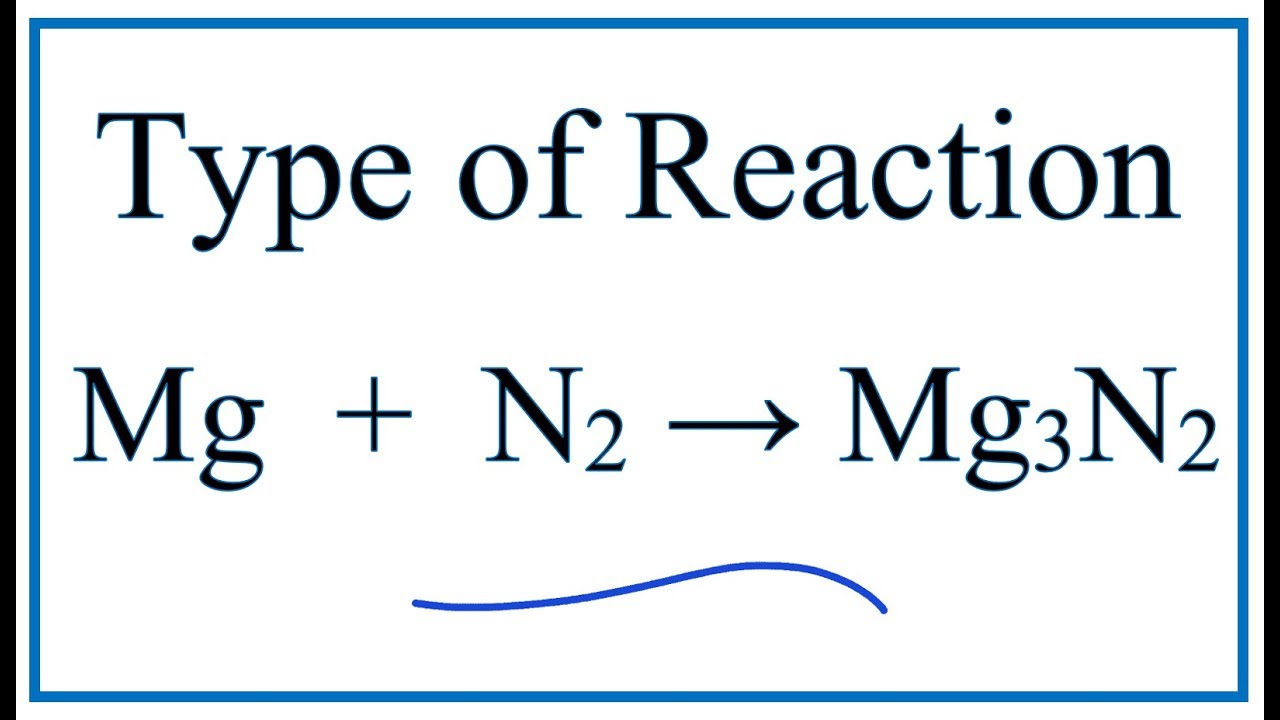
Type Of Reaction For Mg N2 Mg3n2 Youtube

Mg N2 Mg3n2 Balanced Equation Magnesium Plus Nitrogen Balanced Equation Youtube
0 Comments